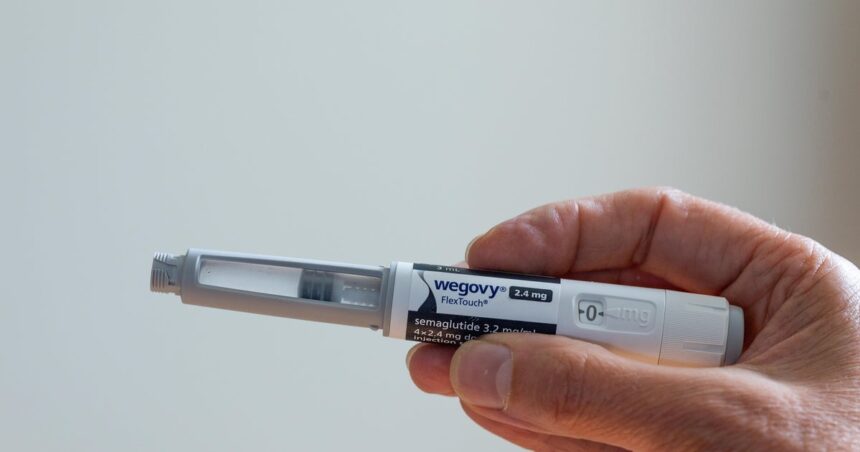
Novo Nordisk remains in a collaboration with Hims & Hers Wellness, asserting the telemedicine business has actually offered a phony variation of the pharmaceutical business’s weight-loss medication Wegovy.
The Danish drugmaker claimed Monday that Wegovy will certainly no more be offered on Hims & hers which it is finishing its cooperation as the San Francisco-based on-line health and wellness business offered “an unlawful duplicate of Wegovy that places client security in jeopardy.”
Novo Nordisk’s transfer to liquify the collaboration is much less than 2 months old, and the firms claimed they are getting in a “long-lasting cooperation” to make weight problems therapies extra obtainable.
Hims & Hers shares dropped greater than $20, or regarding 31%, to $44.10, adhering to the Novo Nordisk statement.
Hims & hers did not instantly react to an ask for remark.
Novo Nordisk claimed it started marketing Wegovy via telemedicine firms consisting of Hims & Hers after an across the country medication lack. Novo Nordisk claimed it wants to move people from utilizing Wegovy’s “combinant, substance variation” to the Food and Medication Administration-approved Semaglutide medication when it involves providing the medication via even more sellers.
” Hims & Hers Health And Wellness, Inc. has actually fallen short to abide by the legislation, which restricts mass sales of substance medicines wholesale under the semblance of “customization” mistakes and is spreading out misleading advertising and marketing that places the security of people in jeopardy,” a Novo Nordisk speaker informed CBS MoneyWatch. “It’s inappropriate, which’s why we determined to finish our cooperation.”
The FDA accepted Wegovy for overweight grownups in March 2024 and included it to the expanding market for the weight-loss tablet market, consisting of Ozempic and Mounjaro. Recently, need for GLP-1 substance abuse to deal with diabetic issues and weight-loss has actually risen, with one in 8 American grownups stating they have actually utilized these therapies.
Novo Nordisk claimed at the launch that the business’s examination revealed that the active ingredients of common medicines offered by telemedicine entities and substance drug stores were generated by international providers in China, and the FDA had actually never ever taken a look at a a great deal of them.
” united state people ought to not be revealed to common medicines made from risky and prohibited international active ingredients,” Novo Nordisk claimed.
Novo Nordisk will certainly remain to market Wegovy on various other telemedicine systems, Dave Moore, executive vice head of state of Novo Nordisk, claimed in a business declaration. Amongst telemedicine firms, Novo Nordisk companions are Lifemd and Ro.